Description
![]()
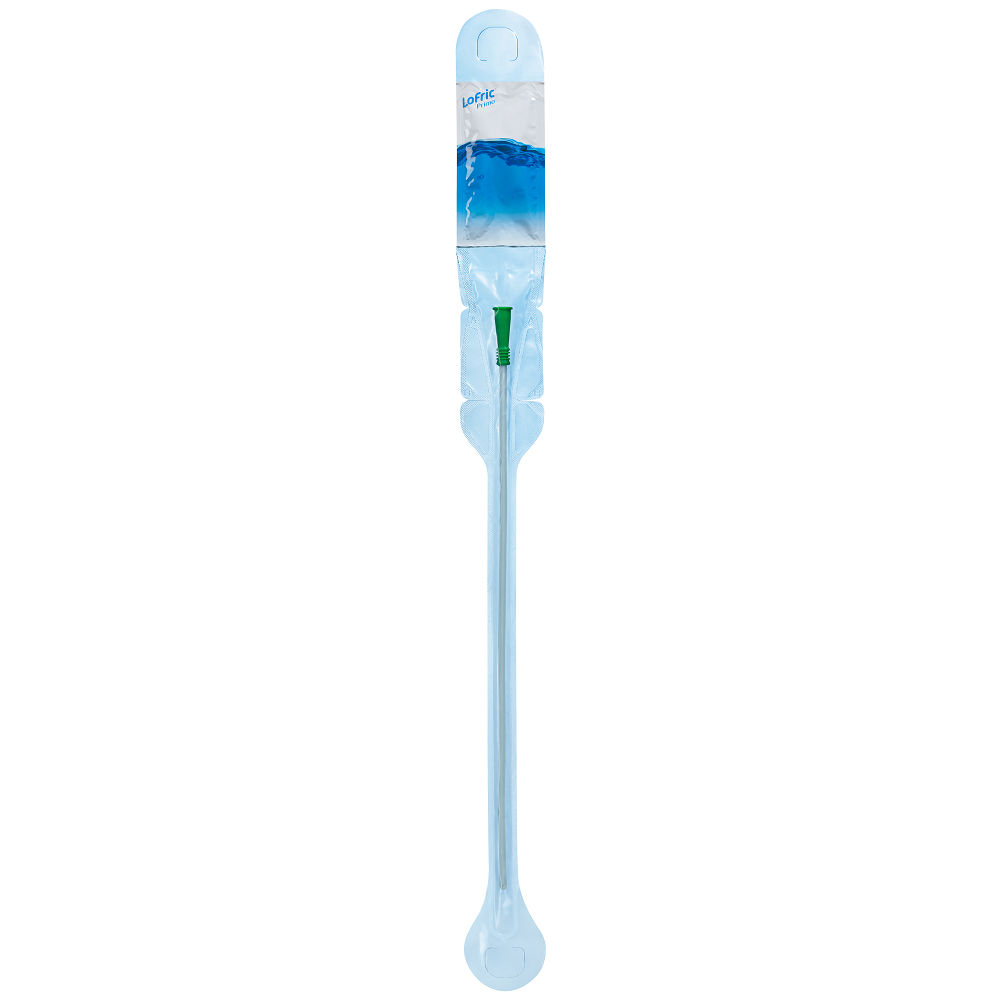
LoFric® is the world’s first hydrophilic intermittent catheter. The LoFric® Primo™ is packaged with its own sterile water – you can safely catheterize even where there is no clean water source available. The packaging keeps the water separate From the catheter until activation so it is easy to fold and discreetly carry in your purse or pocket. LoFric Primo is a perfect solution for people who are active and out of the home Frequently. It’s specially designed coating results in comfort by maintaining the slippery coating throughout the entire catheterization. LoFric is the only catheter proven in long-term clinical studies to reduce the risk of UTIs and other complications when compared to conventional catheters. LoFric Primo is available in sizes for men, women, and children and is latex- Free.
LoFric® PrimoTM is packaged with its own sterile water – you can safely catheterize even where there is no clean water source available. The packaging keeps the water separate from the catheter until activation. Easy to fold. LoFric Primo is a perfect solution for people who are active and out of the home frequently. Available in sizes for men, women, and children.
• Straight-Tip (Nelaton)
• Large loop for opening or hanging
• Adhesive area on back for hanging up the package.
• Sterile water included. Press water pocket to activate.
• Insertion guide. A firm grip without needing to touch the catheter with your fingers.
• Urotonic™ Surface Technology for low friction and maximal comfort.
• Smooth catheter eyes.
• Foldable and discreet.
• Connector has different colors depending on the diameter of the catheter. Can be attached to a standard urine collection bag or extension line.
LoFric Catheters are scientifically proven to show:
• 0% trauma after 5-9 years with LoFric. 1
• 10-20 times lower expected risk of strictures. 2
• 64% lower expected risk of UTIs. 3
• 48% lower expected risk of hematuria. 4
References:
- Waller et al. J Urol 1995;153.345-8
- Li et al. Arch Phys Med Rehabil. 2013;94:782-7
- Hakansson et al. Urol Nurs. 2015;35:239-47
- Rognoni and Tarricone. Health Technology Assessment (HTA), CERGAS Bocconi University 2016




Reviews
There are no reviews yet.